Stop leaking without stopping your life
An innovative endo-urethral medical device
for the treatment of stress urinary incontinence
Benefits




Product
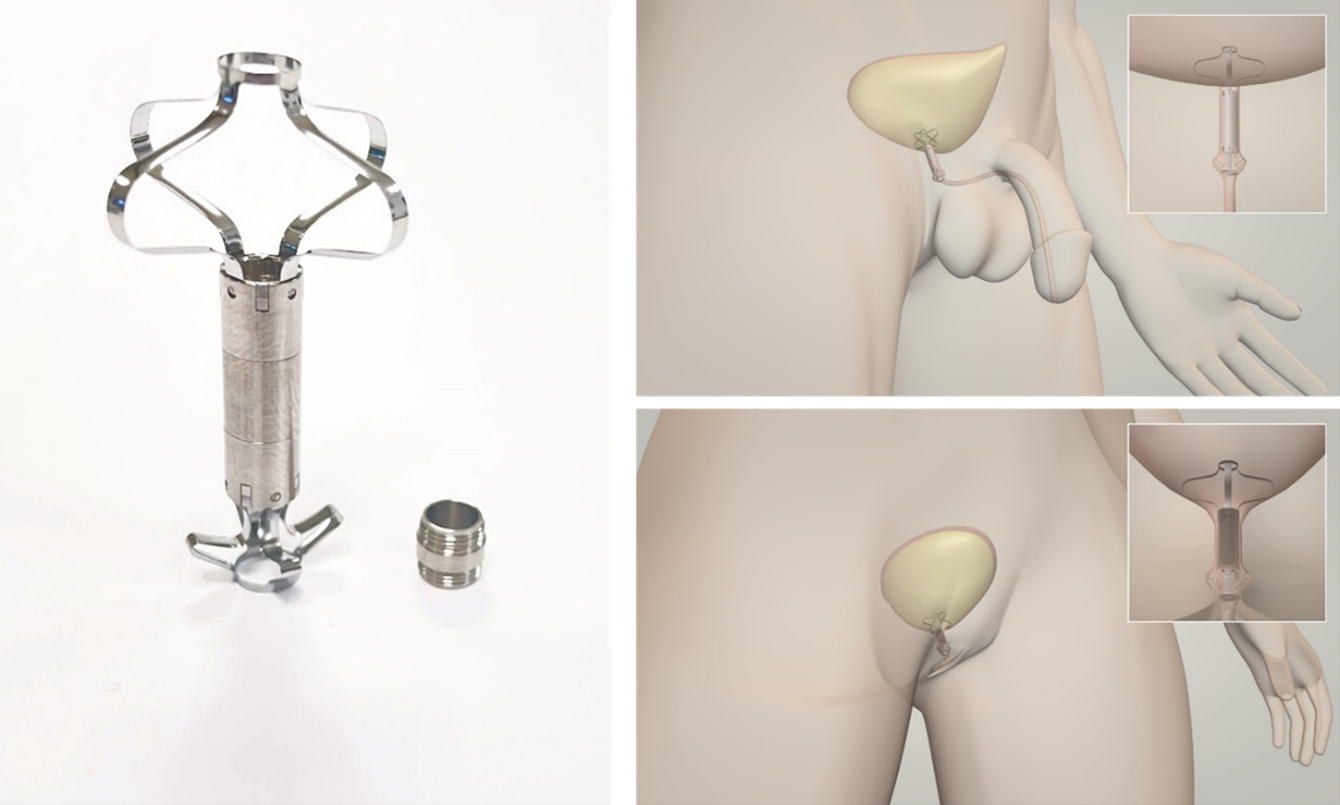
Relief S.r.l. has developed UroRelief, a sterile, single-use, and unisex endourethral medical device that is positioned on the bladder neck, bypassing the urethral sphincter, for treatment of moderate and severe SUI. The device is designed to be inserted in the urethra's natural orifice by means of a routine endoscopic procedure which can be performed during standard urological examinations. The device fully disappears within the body, with no visible parts outside, and it can fully restore control over urination.
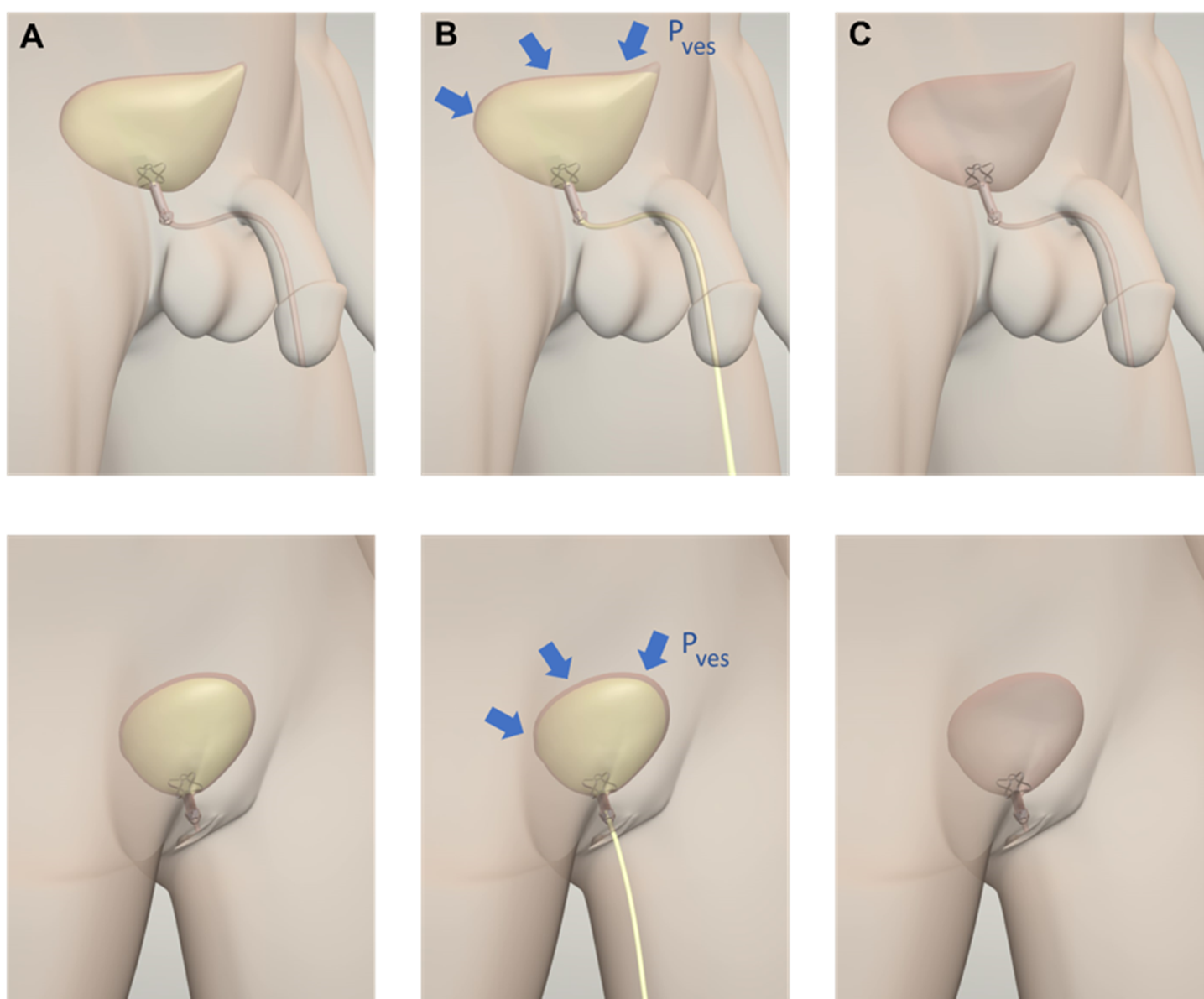
The key element of the device is a silicone valve that replaces the function of the biological external urinary sphincter. The device is activated when the patient contracts the abdomen. The valve is incorporated in a Titanium frame that has the function of ensuring the proper anchoring and stability of the device once inserted. The Nitinol stents collapse to allow the insertion of UroRelief. Once inserted, the valve is normally in a closed position. In this configuration, the device restores continence up to a physiological vesical pressure imposed by the valve. When a patient feels the urge to urinate, he or she applies vesical pressure by contracting the abdomen. The vesical pressure shall be above the valve opening pressure; in this way, the device opens allowing a physiological voiding flow. During the voiding, the valve opening pressure decreases its resistance, making the voiding flow more comfortable and as similar as possible to the physiological one. Once the urination has ended, as well as the pressure imposed by the patient, the device’s valve automatically closes restoring continence to the starting valve opening pressure.

If you are a patient

If you are a physician
Accomplishment
Description
About us
Relief srl was born in March 2019 and the headquarters are located in Pisa (Italy). The company's vision is to become the worldwide leader for the treatment of moderate/severe stress urinary incontinence. The company's mission aims to market a patented, simple, more effective and safer technology than the existing competitors. Our philosophy is to develop efficient and innovative products by keeping in the center of the process the needs of patients and medical doctors by improving significantly the quality of the life of these patients.

Leonardo Ricotti
Associate Professor in BioRobotics